Abstract
Background
Minimal/measurable residual disease (MRD) testing is increasingly utilised and accepted as standard of care to manage a range of different haematological malignancies. It's use as a surrogate outcome in clinical trials of new therapies is being explored, where it has the potential to accelerate drug assessment and approval. The phenotypic and genetic heterogeneity of acute myeloid leukaemia (AML) has limited the use of MRD in this context; however, the European LeukaemiaNet (ELN) MRD working group have recently published consensus guidelines to standardise both flow cytometric and molecular genetic MRD testing. To assess the accuracy of testing and concordance between laboratories, crucial to patient safety, external quality assessment (EQA)/proficiency testing (PT) is required.
Aims
To determine the performance of molecular methods for measuring of MRD using the t(8;21)(q22:q22) RUNX1-RUNX1T1, inv(16)(p13q22) CBFB-MYH11, t(15;17)(q24.1;q21.2) PML-RARA and NPM1 Type A markers in an international interlaboratory study.
Methods
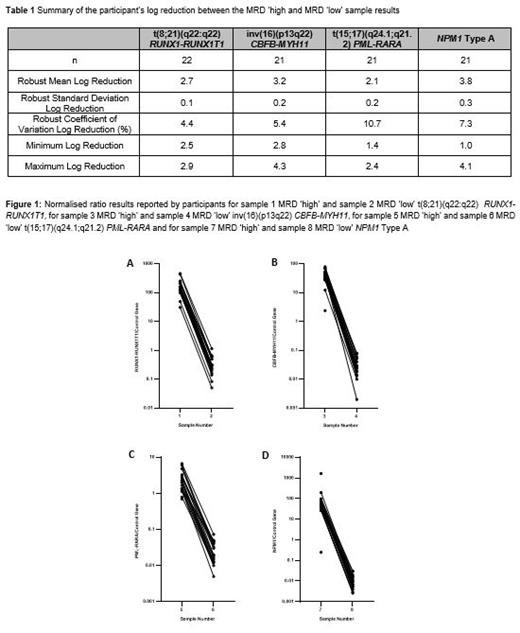
A total of 12 batches of lyophilised EQA material were manufactured. These consisted of three batches of samples for each marker all containing 9x10^6 cells: an MRD 'high' sample; an MRD 'low' sample; and an MRD 'negative' sample. The t(8;21)(q22:q22) RUNX1-RUNX1T1 positive samples were manufactured using the KASUMI-1 cell line, the inv(16)(p13q22) CBFB-MYH11 positive samples using the ME-1 cell line; t(15;17)(q24.1;q21.2 PML-RARA positive samples using the NB4 cell line and the NPM1 Type A (NM_002520.6:c.860_863dup) positive samples using the OCI-AML3 cell line. MRD positive samples were diluted with HL60 cells to achieve the desired MRD level. MRD negative samples were manufactured using the HL60 cell line. The samples were shipped at ambient temperature to the 29 laboratories in 12 countries. Participants were asked to test the blinded samples with their in-house assay and report % normalised ratio of the relevant marker alongside additional methodological and technical data.
Results
For t(8;21) RUNX1-RUNX1T1, all participants who returned results (n=23) classified the MRD 'high' and MRD 'low' samples as positive and the MRD 'negative' sample as negative. The robust mean log reduction between the MRD 'high' and MRD low sample was 2.7 (range 2.5-2.9). For inv(16) CBFB-MYH11, all participants who returned results (n=22) classified the MRD 'high' sample as positive, 21/22 (95.5%) classified the MRD 'low' sample as positive and 21/22 (95.5%) classified the MRD negative sample as negative. The robust mean log reduction between the MRD 'high' and MRD 'low' sample was 3.16 (range 2.8-4.2). For t(15;17) PML-RARA, all participants who returned results (n=22) classified the MRD 'high' sample as positive, 21/22 (95.5%) classified the MRD 'low' sample as positive and 21/22 (95.5%) classified the MRD negative sample as negative. The robust mean log reduction between the MRD 'high' and MRD 'low' sample was 2.1 (range 1.4-2.4). For NPM1, all participants who returned results (n=23) classified the MRD 'high' as positive, 21/23 (91.3%) classified the MRD 'low' sample as positive and, 17/23 (73.4%) classified the MRD negative sample as negative. The robust mean log reduction between the MRD 'high' and MRD 'low' sample was 3.8 (range 3.2-4.2).
Summary/Conclusion
The majority of participants in this study were able to detect and accurately quantify MRD when assessing the t(8;21)(q22:q22) RUNX1-RUNX1T1, inv(16)(p13q22) CBFB-MYH11, t(15;17)(q24.1;q21.2) PML-RARA and NPM1 markers, at levels that would be expected within a clinical trial or standard of care setting. A high proportion of participants reported false positive results in the NPM1 marker negative sample. This would have significant consequences clinically, with NPM1 marker false-positivity potentially committing patients to unneeded additional chemotherapy and/or transplant with the attendant risk of morbidity and mortality which highlights the need for ongoing EQA in this area. UK NEQAS LI will work with laboratories advocating they undertake a root cause analysis process to identify the source(s) of error contributing to false positive NPM1 marker results and support their subsequent corrective actions; sharing any educational findings with all participants.
Scott: Novartis: Research Funding; Biorad: Research Funding. Dillon: Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Research Support, Educational Events; Amgen: Other: Research support (paid to institution); Astellas: Consultancy, Other: Educational Events , Speakers Bureau; Menarini: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Session chair (paid to institution), Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: educational events; Jazz: Other: Education events; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees. Whitby: Roche: Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Other: Teaching.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal